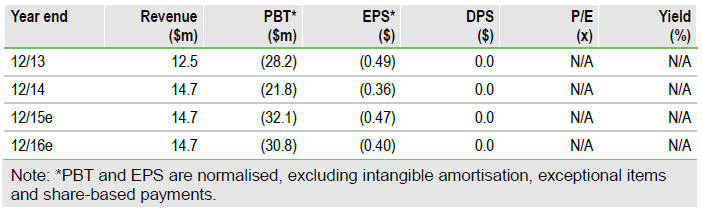
Broadening hypoxia focus with TH-4000
Preclinical and Phase I data on Threshold Pharmaceuticals Inc (NASDAQ:THLD)'s newest hypoxia asset TH-4000 were recently presented at AACR. Threshold now plans to move TH-4000 into Phase II trials in both lung and head and neck cancers. At this stage we do not formally include TH-4000 in our unchanged $949m valuation, although TH-4000 could address sizeable markets with often limited treatment options. The major catalysts for Threshold remain related to evofosfamide, where the number of events needed for analysis of the Phase III STS and pancreatic trials should be reached in H215.
Introducing TH-4000 at AACR
TH-4000 is a hypoxia-activated EGFR-TKI, which was in-licensed in September 2014 and has already completed a Phase I trial. Unlike other EGFR-TKI drugs such as erlotinib and afatinib, which are limited by rash and diarrhoea, TH-4000 was not associated with dose-limiting rash in the Phase I trial. Furthermore, preclinical data suggest that TH-4000 could have activity in certain subsets of lung cancer patients with poor prognosis where current TKI therapy has limited benefit.
Phase II trials in both lung and head and neck cancer
Threshold is planning to start Phase II trials in both a subset of lung cancer and in head and neck (H&N) cancer. Progression to Phase II is supported by both the Phase I and preclinical data package presented at AACR. We expect both trials to start in the coming months, funded by the recent capital increase.
Evofosfamide trials approaching key events in H215
Threshold’s lead asset evofosfamide (TH-302) is currently being investigated in Phase III trials in STS (soft tissue sarcoma) and pancreatic cancer. Both are event-driven trials and based on the current rate, Threshold expects the number of events to trigger full analysis to be reached in H215, with top-line data available shortly after. Both indications represent blockbuster market opportunities for evofosfamide.
To Read the Entire Report Please Click on the pdf File Below