Pfizer Inc. (NYSE:PFE) along with Germany-based partner Merck KGaA announced positive data from interim analysis of a phase III study evaluating their PD-L1 inhibitor Bavencio (avelumab). The JAVELIN Bladder 100 study is evaluating Bavencio plus best supportive care (“BSC”) as a first-line maintenance therapy, following induction chemotherapy in patients with locally advanced/metastatic urothelial carcinoma, a form of bladder cancer, (“UC”) compared to BSC alone. The study met its primary endpoint of overall survival (“OS”).
Interim data from the study showed that patients in the Bavencio arm achieved statistically significant improvement in OS compared to patients receiving BSC alone, current standard-of-care. Pfizer stated in the press release that Bavencio becomes the first immunotherapy to significantly prolong OS as first-line maintenance treatment for advanced urothelial carcinoma. Detailed data from study will be presented at any upcoming medical congress and submitted to the FDA for approval.
In 2017, Bavnecio had received accelerated approval in the United States as a treatment for locally advanced or metastatic UC with disease progression on or after platinum-containing chemotherapy or who had disease progression within 12 months of treatment with a platinum-containing neoadjuvant or adjuvant chemotherapy regimen. Data from the JAVELIN Bladder 100 study will also support Bavencio’s continued approval in the indication.
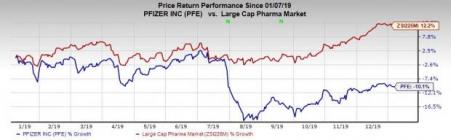
Shares of Pfizer have decreased 10.1% in the past year against the industry’s rise of 12.2%.
Notably, Bavencio is one of the key candidates in Pfizer’s oncology pipeline. Per the company, the Bavencio development program (known as JAVELIN) involves more than 10,000 patients currently being investigated across 15 tumor types including head and neck, non-small cell lung, urothelial and others. Although Bavencio is currently approved for three relatively smaller indications, it might emerge as a key long-term growth driver for Pfizer on future label expansion approvals.
Bavencio had a mixed 2019. While the drug failed in two clinical studies evaluating it in gastric and ovarian cancer, it was approved by the FDA for treating kidney cancer in combination with Pfizer’s Inlyta.
Bavencio faces stiff competition from other approved PD-L1 inhibitors in the market, such as Merck’s (NYSE:MRK) blockbuster drug Keytruda (pembrolizumab), Bristol-Myers’ (NYSE:BMY) Opdivo (nivolumab) and AstraZeneca’s (NYSE:AZN) Imfinzi (durvalumab).
Zacks Rank
Pfizer currently carries a Zacks Rank #3 (Hold). You can see the complete list of today’s Zacks #1 Rank (Strong Buy) stocks here.
Just Released: Zacks’ 7 Best Stocks for Today
Experts extracted 7 stocks from the list of 220 Zacks Rank #1 Strong Buys that has beaten the market more than 2X over with a stunning average gain of +24.6% per year.
These 7 were selected because of their superior potential for immediate breakout.
See these time-sensitive tickers now >>
Merck & Co., Inc. (MRK): Free Stock Analysis Report
AstraZeneca PLC (AZN): Free Stock Analysis Report
Bristol-Myers Squibb Company (BMY): Free Stock Analysis Report
Pfizer Inc. (PFE): Free Stock Analysis Report
Original post
Zacks Investment Research