Orexo (OTC:ORXOY) has announced a partnership for the digital therapy of opioid use disorder (OUD) with GAIA, a global leader in digital therapeutics. The partnership will use GAIA’s expert system technology platform, broca, to develop a treatment management and support a product tailored to individual OUD patients.
Orexo moves ‘beyond the pill’
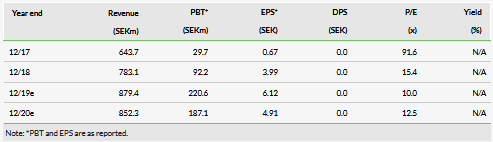
Orexo’s newly announced partner, GAIA, is well established in digital therapeutics (DTx), with products already approved for use in the treatment of depression, other neurological disorders, multiple sclerosis and epilepsy in real-world practice and proven in clinical trials. This step aligns Orexo with some of the bigger global pharmaceutical companies that have already moved ‘beyond the pill’ to the management of patients with difficult-to-treat indications, where patient compliance is challenging and digital therapy allows treatment to be differentiated. All these factors come into play in OUD patients where relapse is common, and the diagnosis brings social acceptance and exclusion issues. An artificial intelligence (AI) product that supplements face-to-face counselling and medically assisted treatment (MAT), eg Orexo’s Zubsolv, will be developed as OXD-01 and is expected to be launched by Orexo in the US in 2022, subject to regulatory approval. Orexo will be responsible for the clinical development, regulatory approval and commercialisation of OXD-01. As the increase in operational expense is not expected to be material, we have not changed our estimate of Orexo’s FY19 total operational spend (SEK545m). Our valuation is unchanged at SEK3.46bn or SEK98.79 per share, although we recognise that upside now exists as the prescription DTx programme market is expected to be valued at $890m by 2026.
Business description
Orexo is a Swedish speciality pharma company, with expertise in drug delivery/reformulation technologies (in particular, sublingual formulations) and a US commercial infrastructure for its opioid dependence therapy, Zubsolv (marketed by Orexo in the US and being out-licensed to partners ex-US). It also has three other clinical assets including OX124, which has reported positive Phase I results.