Mirati Therapeutics, Inc. (NASDAQ:MRTX) has delivered a solid performance in the second quarter of 2023, with both positive and negative factors impacting its financial results. The company's earnings per share (EPS) on a normalized and GAAP basis have exceeded market expectations, indicating strong performance. Similarly, Mirati reported higher-than-expected revenue, showcasing its growth potential in the biotech sector.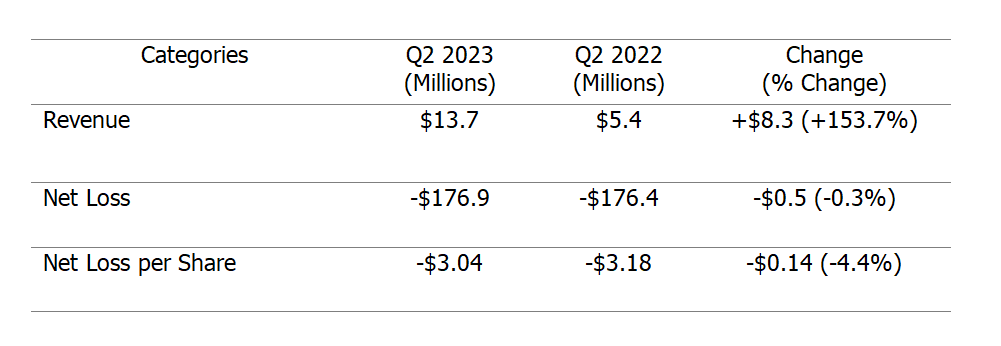
Strategic Drivers & Pipeline
One of the key growth drivers for Mirati is the advancement of its lead drug candidate, adagrasib, in first-line non-small cell lung cancer (NSCLC) patients with KRAS G12C mutation. The positive data from the ongoing Phase 2 study, including a high objective response rate (ORR) and favorable progression-free survival (PFS), support the rapid advancement of adagrasib into a Phase 3 study. This presents a significant market opportunity for Mirati, positioning adagrasib as a potential market leader in this space. Mirati's pipeline also includes MRTX1719, an MTA-cooperative PRMT5 inhibitor, which has shown promising initial clinical data in patients with MTAP-deleted tumors. The drug's unique mechanism of action and favorable safety profile are driving its potential as a first-in-class and best-in-class treatment option for indications such as non-small cell lung cancer, pancreatic cancer, and mesothelioma. The commercial success of KRAZATI, Mirati's approved drug for second-line NSCLC, further contributes to the company's growth. KRAZATI has demonstrated a differentiated efficacy profile and has gained rapid adoption in the market. With broad coverage and minimal access barriers, KRAZATI has the potential to become a market-leading product in the KRAS G12C space.
Buyout Buzz
Mirati Therapeutics (MRTX) shares have historically been sensitive to buyout rumors, with a recent surge of approximately 40% based solely on Sanofi (NASDAQ:SNY)'s potential interest. While these speculations can temporarily boost share prices, they often leave investors in a state of uncertainty, especially when expected deals don't materialize. Such volatility attracts traders who capitalize on the rapid price movements, but for long-term investors, outcomes like the recent Bristol-Myers Squibb's (NYSE:BMY) acquisition can be less than ideal.
Deal Structure Insights- Bristol Myers' Strategic Move
The cash agreement sees Mirati shares valued at $58 each, totaling $4.8B. Additionally, Mirati shareholders will receive a non-tradeable Contingent Value Right (CVR) for every share, potentially amounting to around $1B. To sweeten the deal, a Contingent Value Right (CVR) of $12 per share was introduced, hinging on the FDA's approval of Mirati's drug candidate, MRTX1719, within seven years post-merger. However, the market's hesitance to value the CVR at its full potential of $12 suggests significant skepticism regarding the deal's future benefits. This CVR is contingent upon the FDA's acceptance of Mirati's new drug application for MRTX1719, a treatment for non-small cell lung cancer (NSCLC), within seven years post the deal's closure. The drug, classified as a PRMT5-MTA inhibitor, is slated for Phase 2 trials in the first half of 2024. Bristol Myers has highlighted Mirati’s NSCLC treatment, Krazati, which addresses tumors with KRAS G12C mutations, as a significant factor behind the acquisition. The company also drew attention to two other promising KRAS inhibitors in Mirati's pipeline, MRTX1133 and MRTX0902.
Impact of FDA Decisions
Recent FDA decisions have further complicated the market landscape. Mirati shares experienced a surge following the FDA's unfavorable verdict on Amgen’s Lumakras, a treatment also targeting NSCLC with KRAS G12C mutations, and subsequent news of Sanofi's potential interest in acquiring Mirati. The FDA advisory committee's decision deemed results from a pivotal Lumakras study as unreliable. This decision holds weight as Amgen (NASDAQ:AMGN) intended to utilize this study to gain full Lumakras approval, which had already secured accelerated approval in 2021. In contrast, Mirati’s Krazati was granted accelerated approval in December 2022, and the company is set to unveil data from a vital Phase 3 trial soon. Some analysts believe that potential FDA doubts on Lumakras' efficacy might offer Krazati a larger market share, while others anticipate Lumakras to remain in the market, possibly achieving full FDA approval through alternate regulatory means.
Valuation Concerns
Mirati's financial performance raises eyebrows when considering the company's escalating operational losses and negative free cashflow. Despite the enthusiasm around its FDA-approved drug, KRAZATI, the drug is still in its early revenue-generating stages. Given the challenges in determining a development-stage biotech company's worth, the Mirati case underscores the complexities of merger deal dynamics and their subsequent market impacts.
Market Speculation and Potential Bidders
The acquisition has ignited widespread market speculation, especially regarding the emergence of alternative bidders for Mirati and the probability of the CVR payment coming to fruition. We believe the current deal valuation is appropriate. However, the possibility remains that another contender might step forward, given that Bristol Myers' offer could undervalue Mirati's strategic significance. Merck and Pfizer (NYSE:PFE) emerge as potential bidders, given their capability to maximize the potential of adagrasib and MRTX1719. Still, there's a prevailing skepticism around the realization of the CVR payment. We believe that the uncertainties surrounding the Phase 2 clinical trial outcomes for MRTX1719 present challenges, and the drug could face competition from similar candidates, notably from companies like Amgen.
Based on the analysis of Mirati's performance and growth drivers, we assign to hold on right now and closely monitor the progress of Mirati's ongoing clinical trials, particularly the Phase 3 study for adagrasib in first-line NSCLC and the Phase 2 study for MRTX1719 in MTAP-deleted tumors. Additionally, tracking the market uptake and sales performance of KRAZATI in the second-line NSCLC market will provide insights into the company's growth trajectory.
Disclosure: We don’t hold any position in the stock and this is not a recommendation of any kind as investing carries risk.
Read More Here: https://equisights.com/
