Janssen, a subsidiary of Johnson & Johnson (NYSE:JNJ) , and partner Bayer (DE:BAYGN) AG (OTC:BAYRY) announced that the FDA has approved a label expansion of its blood thinner, Xarelto (rivaroxaban).
With the latest label expansion, Xarelto can now be prescribed for prevention of venous thromboembolism (“VTE”) or blood clot, and VTE-related death during hospitalization and post-hospital discharge in acutely ill medical patients at risk for VTE and not at high risk of bleeding
Xarelto is already approved for a wide range of patient population for prevention and reduction in the risk of recurrence of VTE.
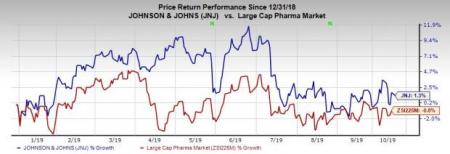
Shares of J&J have increased 1.3% so far this year against the industry’s decrease of 0.8%.
The approval was based on data from a phase III program including MAGELLAN and MARINER studies, which evaluated Xarelto in patients with acute medical illnesses. Previously announced data from the MAGELLAN study showed that Xarelto was non-inferior to Sanofi’s (NASDAQ:SNY) Lovenox (enoxaparin) in short-term use, and superior in long-term use compared to short-term enoxaparin treatment followed by placebo.
Although data from the MARINER study did not meet its primary endpoint, Xarelto achieved positive benefit-risk profile based on reduction in symptomatic VTE.
Per the press release, more than seven million people are hospitalized in the United States for acute medical illness. These patients have increased risk of blood clot during three months of discharge from hospitals. Several marketed anti-coagulants are administered through injection and mainly during hospitalization. Xarelto, which is an orally administered anti-coagulant, will provide a better treatment option for these patients and can be administered for a few days after discharge as well. Label expansion of Xarelto for use in hospitalized acutely ill medical patients is likely to create an incremental growth opportunity.
Meanwhile, J&J continues to evaluate Xarelto in other patient population. In July, the company announced that the drug has achieved similar low risk of recurrent VTE or blood clots and similar rates of bleeding compared to current standard anticoagulation therapy in pediatric patients in phase III EINSTEIN-Jr study.
Another popular blood thinner, Eliquis, is marketed by Bristol-Myers (NYSE:BMY) in collaboration with Pfizer (NYSE:PFE).
Zacks Rank
J&J currently carries a Zacks Rank #4 (Sell).
You can see the complete list of today’s Zacks #1 Rank (Strong Buy) stocks here.
Today's Best Stocks from Zacks
Would you like to see the updated picks from our best market-beating strategies? From 2017 through 2018, while the S&P 500 gained +15.8%, five of our screens returned +38.0%, +61.3%, +61.6%, +68.1%, and +98.3%.
This outperformance has not just been a recent phenomenon. From 2000 – 2018, while the S&P averaged +4.8% per year, our top strategies averaged up to +56.2% per year.
See their latest picks free >>
Bayer Aktiengesellschaft (BAYRY): Free Stock Analysis Report
Sanofi (PA:SASY) (SNY): Free Stock Analysis Report
Bristol-Myers Squibb Company (BMY): Free Stock Analysis Report
Johnson & Johnson (JNJ): Free Stock Analysis Report
Original post