Ablynx (ABLX.BR) recently reported promising data from the Phase II open-label extension study with ATN-103 in rheumatoid arthritis (RA), which could reignite interest in the product among major pharma companies. But even if this Nanobody is only partnered with regional companies, or not at all, Ablynx still offers an attractive investment. Phase II data on ALX-0061 in RA and Phase I data on the inhaled ALX-0171 are due in H212. Ablynx could also form new alliances as the benefits and capabilities of Nanobodies become more apparent. We have raised our valuation by €31m to €479m.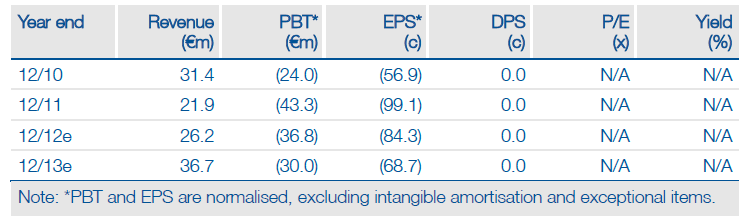
New data on ATN-103 increase licensing prospects
Data from the Phase II open-label extension (OLE) study (n=266) in RA suggest that ATN-103’s safety and efficacy is at least comparable to that of other TNFα inhibitors and could even be better, because of lower immunogenicity. Ablynx hopes that the new data, together with the low production costs, stability and potential dosing advantages, will convince a major pharma company to in-license ATN-103. Ablynx will provide an indication on how partnering discussions are proceeding by the year end.
Clinical data on ALX-0061 and ALX-0171 in H212
Proof-of-concept Phase II data on ALX-0061 (anti-IL-6R) in RA and results from the Phase I with Ablynx’s first inhaled Nanobody, ALX-0171 (anti-RSV), are due in H212. The former could result in ALX-0061 being partnered. There could be much interest in this product because of Roche’s data on Actemra, which also targets IL-6R, that showed that Actemra has significant advantages over TNFα inhibitors in RA.
Unique platform and deals
The strengths of Ablynx’s Nanobody platform are becoming clearer, which could result in Ablynx forming more partnerships. Novartis is about to start a Phase I study with TAS266 (anti-DR5). Previous attempts to activate DR5 with monoclonal antibodies have been unsuccessful. ALX-0171 shows that Nanobodies do not need to be injected. The low immunogenicity observed in the OLE trial should remove any worries about using a llama-derived product.
Valuation: €479m based on DCF
We have increased our valuation from €448m to €479m. If ATN-103 is excluded, we value Ablynx at €260m, suggesting that there is considerable upside to the current market cap of €123m, even if Ablynx is unable to partner ATN-103. The company has a strong balance sheet and could operate into 2015 without raising capital.
To Read the Entire Report Please Click on the pdf File Below.
- English (UK)
- English (India)
- English (Canada)
- English (Australia)
- English (South Africa)
- English (Philippines)
- English (Nigeria)
- Deutsch
- Español (España)
- Español (México)
- Français
- Italiano
- Nederlands
- Português (Portugal)
- Polski
- Português (Brasil)
- Русский
- Türkçe
- العربية
- Ελληνικά
- Svenska
- Suomi
- עברית
- 日本語
- 한국어
- 简体中文
- 繁體中文
- Bahasa Indonesia
- Bahasa Melayu
- ไทย
- Tiếng Việt
- हिंदी
Ablynx OutlookL Start Of An Important 6 Months
Published 07/22/2012, 05:54 AM
Updated 07/09/2023, 06:31 AM
Ablynx OutlookL Start Of An Important 6 Months
Start of an important six months
3rd party Ad. Not an offer or recommendation by Investing.com. See disclosure here or
remove ads
.
Latest comments
Install Our App
Risk Disclosure: Trading in financial instruments and/or cryptocurrencies involves high risks including the risk of losing some, or all, of your investment amount, and may not be suitable for all investors. Prices of cryptocurrencies are extremely volatile and may be affected by external factors such as financial, regulatory or political events. Trading on margin increases the financial risks.
Before deciding to trade in financial instrument or cryptocurrencies you should be fully informed of the risks and costs associated with trading the financial markets, carefully consider your investment objectives, level of experience, and risk appetite, and seek professional advice where needed.
Fusion Media would like to remind you that the data contained in this website is not necessarily real-time nor accurate. The data and prices on the website are not necessarily provided by any market or exchange, but may be provided by market makers, and so prices may not be accurate and may differ from the actual price at any given market, meaning prices are indicative and not appropriate for trading purposes. Fusion Media and any provider of the data contained in this website will not accept liability for any loss or damage as a result of your trading, or your reliance on the information contained within this website.
It is prohibited to use, store, reproduce, display, modify, transmit or distribute the data contained in this website without the explicit prior written permission of Fusion Media and/or the data provider. All intellectual property rights are reserved by the providers and/or the exchange providing the data contained in this website.
Fusion Media may be compensated by the advertisers that appear on the website, based on your interaction with the advertisements or advertisers.
Before deciding to trade in financial instrument or cryptocurrencies you should be fully informed of the risks and costs associated with trading the financial markets, carefully consider your investment objectives, level of experience, and risk appetite, and seek professional advice where needed.
Fusion Media would like to remind you that the data contained in this website is not necessarily real-time nor accurate. The data and prices on the website are not necessarily provided by any market or exchange, but may be provided by market makers, and so prices may not be accurate and may differ from the actual price at any given market, meaning prices are indicative and not appropriate for trading purposes. Fusion Media and any provider of the data contained in this website will not accept liability for any loss or damage as a result of your trading, or your reliance on the information contained within this website.
It is prohibited to use, store, reproduce, display, modify, transmit or distribute the data contained in this website without the explicit prior written permission of Fusion Media and/or the data provider. All intellectual property rights are reserved by the providers and/or the exchange providing the data contained in this website.
Fusion Media may be compensated by the advertisers that appear on the website, based on your interaction with the advertisements or advertisers.
© 2007-2024 - Fusion Media Limited. All Rights Reserved.