AbbVie Inc. (NYSE:ABBV) announced that its investigational oral JAK-1 selective inhibitor, upadacitinib, met the primary endpoints in a phase III study from its SELECT program, which evaluated the candidate for the treatment of patients with rheumatoid arthritis (“RA”).
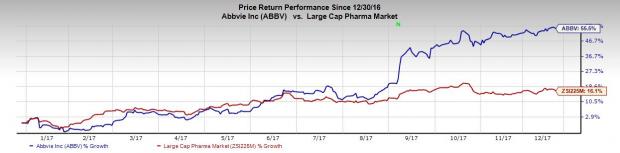
AbbVie’s shares have outperformed the industry so far this year. The stock has surged 55.5% compared with the industry’s 16.1% rally.
The phase III study — SELECT-MONOTHERAPY — evaluated upadacitinib monotherapy in moderate to severe RA patients who were not responsive to methotrexate for a period of 14-weeks. Both the once daily doses, 15 mg and 30 mg, evaluated in the study demonstrated superiority in ACR20 response and low disease activity (LDA) compared to methotrexate.
The ACR20 response was achieved in 68% of the patients receiving 15 mg dose while the response rate was 71% for the 30 mg dose versus 41% for methotrexate. The LDA was achieved in 41%, 28% and 8% of patients receiving 30 mg, 15 mg or methotrexate, respectively.
Upadacitinib is being evaluated in a large program, which includes six studies on RA patients. AbbVie is also developing the candidate in psoriatic arthritis, Crohn's disease, ulcerative colitis, ankylosing spondylitis and atopic dermatitis.
The candidate has also met endpoints in two phase III studies from the SELECT program. The SELECT-MONOTHERAPY study supported the potential of the candidate in treating RA patients without a background methotrexate therapy.
Successful development of upadacitinib will boost AbbVie’s RA portfolio, which already includes the blockbuster drug, Humira. Sales of Humira ($13.5 billion) accounted for 66% of net revenues in the first nine months of 2017.
The approval of Incyte Corporation (NASDAQ:INCY) / Eli Lilly and Company’s (NYSE:LLY) Olumiant in Europe in February 2017 has not had any significant impact on Humira’s sales, as it is approved in patients who are unresponsive to TNF inhibitors like Humira. Moreover, Olumiant’s new drug application received a complete response letter from the FDA in April this year. Meanwhile, upadacitinib may be in direct line of competition with Olumiant as both are JAK-inhibitors.
Zacks Rank & Stock to Consider
AbbVie carries a Zacks Rank #3 (Hold).
Celldex Therapeutics, Inc. (NASDAQ:CLDX) is a better-ranked stock in the pharma sector, carrying a Zacks Rank #2 (Buy). You can see the complete list of today’s Zacks #1 Rank (Strong Buy) stocks here.
Celldex’s loss estimates narrowed from 93 cents to 90 cents for 2017 and from 90 cents to 89 cents over the last 30 days. The company delivered a positive earnings surprise in three of the trailing four quarters with an average beat of 15.36%.
Zacks’ Best Private Investment Ideas
While we are happy to share many articles like this on the website, our best recommendations and most in-depth research are not available to the public.
Starting today, for the next month, you can follow all Zacks' private buys and sells in real time. Our experts cover all kinds of trades… from value to momentum . . . from stocks under $10 to ETF and option moves . . . from stocks that corporate insiders are buying up to companies that are about to report positive earnings surprises. You can even look inside exclusive portfolios that are normally closed to new investors.
Click here for Zacks' private trades >>
Eli Lilly and Company (LLY): Free Stock Analysis Report
AbbVie Inc. (ABBV): Free Stock Analysis Report
Incyte Corporation (INCY): Free Stock Analysis Report
Celldex Therapeutics, Inc. (CLDX): Free Stock Analysis Report
Original post
Zacks Investment Research