BioInvent's (BINV.ST) FY12 results showed revenues and grants of SEK55.5m with costs of SEK246.6m, including SEK49.2m of provisions and restructuring costs. The company has restructured to give a stated cost base of SEK75m before revenues in 2013. There was SEK100m of cash on 31 December. The BI-505 Phase I dose-escalating and safety study in relapsed and refractory multiple myeloma (MM) indicated a dose of 10mg/kg. BI-505 will progress to a small Phase IIa during 2013. BioInvent intends to partner BI-505 in 2014. Two preclinical antibodies have entered development.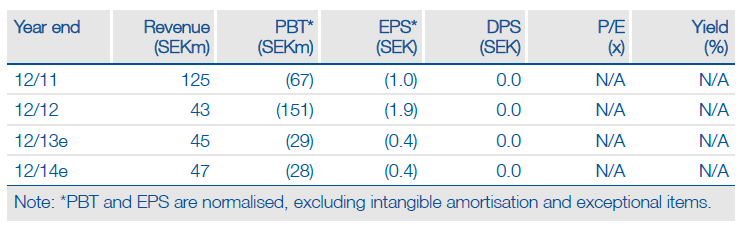
Rebuilding the portfolio: Two new projects
A product, ADC-1013, is a co-development with Alligator Bioscience, also a Lund (Sweden) biotechnology company. A deal was done in August 2012 combining Alligator’s Fragment INduced Diversity (FIND) technology with BioInvent’s n-CoDeR library. The target is undisclosed, but may be similar to Yervoy (ipilimumab, BMS) to stimulate an anti-cancer immune response. Toxicology will be carried out in 2013 enabling Phase I in 2014-15. An in-house development is BI-1206 against CD32b. This inhibits B-cell activity so may be effective in non-Hodgkin’s Lymphoma, where Ritriuxan (Rituximab) is the leading antibody therapy. Macrogenics had a CD32b project in the mid-2000s, but this did not progress. BI-1206 may enter toxicity testing in 2014. TB-403 has recently shown promise in medulloblastoma.
BI-505 progression to Phase II
BioInvent has evidence that BI-505 (which binds to intercellular adhesion molecule ICAM-1) triggers apoptosis (cell suicide) in MM cells. BI-505 also seems to enable immune cell-dependent killing of MM cells. BI-505 at 10mg/kg saturates the ICAM sites on bone marrow myeloma cells; this dose will be used for a small Phase II in asymptomatic MM due to start in 2013, with a deal expected in 2014. The likelihood of success remains at 30%.
Valuation: Cash conservation while building the portfolio
BioInvent had year-end cash of SEK100m. If 2013 revenues are SEK45m or better with costs of SEK75m, the company has cash into 2014. The small Phase IIa BI-505 study due to start in 2013 will be inexpensive followed by a deal assumed at a 50% probability and $10m upfront with a 15% royalty; doing good-value deals at an early development stage can be difficult. Collaborations may generate milestones in 2013 if partners designate clinical candidates. A revised indicative value indicates SEK3.20 per share. This uses a 5-fold multiple to reflect the emerging, but unproven, portfolio.
To Read the Entire Report Please Click on the pdf File Below.
- English (UK)
- English (India)
- English (Canada)
- English (Australia)
- English (South Africa)
- English (Philippines)
- English (Nigeria)
- Deutsch
- Español (España)
- Español (México)
- Français
- Italiano
- Nederlands
- Português (Portugal)
- Polski
- Português (Brasil)
- Русский
- Türkçe
- العربية
- Ελληνικά
- Svenska
- Suomi
- עברית
- 日本語
- 한국어
- 简体中文
- 繁體中文
- Bahasa Indonesia
- Bahasa Melayu
- ไทย
- Tiếng Việt
- हिंदी
BioInvent International: BI-505 First Clinical Data
Published 03/18/2013, 01:11 AM
Updated 07/09/2023, 06:31 AM
BioInvent International: BI-505 First Clinical Data
Focused on cancer
Latest comments
Loading next article…
Install Our App
Risk Disclosure: Trading in financial instruments and/or cryptocurrencies involves high risks including the risk of losing some, or all, of your investment amount, and may not be suitable for all investors. Prices of cryptocurrencies are extremely volatile and may be affected by external factors such as financial, regulatory or political events. Trading on margin increases the financial risks.
Before deciding to trade in financial instrument or cryptocurrencies you should be fully informed of the risks and costs associated with trading the financial markets, carefully consider your investment objectives, level of experience, and risk appetite, and seek professional advice where needed.
Fusion Media would like to remind you that the data contained in this website is not necessarily real-time nor accurate. The data and prices on the website are not necessarily provided by any market or exchange, but may be provided by market makers, and so prices may not be accurate and may differ from the actual price at any given market, meaning prices are indicative and not appropriate for trading purposes. Fusion Media and any provider of the data contained in this website will not accept liability for any loss or damage as a result of your trading, or your reliance on the information contained within this website.
It is prohibited to use, store, reproduce, display, modify, transmit or distribute the data contained in this website without the explicit prior written permission of Fusion Media and/or the data provider. All intellectual property rights are reserved by the providers and/or the exchange providing the data contained in this website.
Fusion Media may be compensated by the advertisers that appear on the website, based on your interaction with the advertisements or advertisers.
Before deciding to trade in financial instrument or cryptocurrencies you should be fully informed of the risks and costs associated with trading the financial markets, carefully consider your investment objectives, level of experience, and risk appetite, and seek professional advice where needed.
Fusion Media would like to remind you that the data contained in this website is not necessarily real-time nor accurate. The data and prices on the website are not necessarily provided by any market or exchange, but may be provided by market makers, and so prices may not be accurate and may differ from the actual price at any given market, meaning prices are indicative and not appropriate for trading purposes. Fusion Media and any provider of the data contained in this website will not accept liability for any loss or damage as a result of your trading, or your reliance on the information contained within this website.
It is prohibited to use, store, reproduce, display, modify, transmit or distribute the data contained in this website without the explicit prior written permission of Fusion Media and/or the data provider. All intellectual property rights are reserved by the providers and/or the exchange providing the data contained in this website.
Fusion Media may be compensated by the advertisers that appear on the website, based on your interaction with the advertisements or advertisers.
© 2007-2024 - Fusion Media Limited. All Rights Reserved.