Pfizer Stock Is Ready To Be Added To Your Portfolio
MarketBeat.com | Oct 27, 2021 02:05AM ET
Pharmaceutical giant Pfizer (NYSE:PFE)'s stock may have finally put in a bottom in the post-pandemic sell-off. At time of writing, the Company was awaiting feedback from the U.S. Food and Drug Administration (FDA) on its COVID-19 vaccine for children as well as booster shots for adults. Both of these catalysts would bolster top and bottom-line growth if approved, adding a second life to shares that initially rallied on its initial COVID-19 vaccine approval and distribution.
The reopening trend relies almost solely on the continued acceleration of COVID vaccinations. The growing need for booster shots will extend the longevity of Pfizer’s COVID treatments providing more upside visibility for investors. Prudent investors seeking exposure in the next leg up on Pfizer should watch for opportunistic pullbacks in shares.
Q2 FY 2021 Earnings Release
On July 28, 2021, Pfizer released its fiscal second-quarter 2021 results for the quarter ending June 2021. The Company reported an earnings-per-share (EPS) profit of $1.07 versus $0.98 consensus analyst estimates, a $0.09 beat. The Company saw revenues grow 60.8% year-over-year (YoY) to $18.98 billion beating analyst estimates for $18.72 billion.
Full-Year 2021 Guidance Raise
Pfizer raised its full-year 2021 estimates for EPS coming in between $3.95 to $4.00 versus $3.65 consensus analyst estimates. Full-year 2021 revenues are expected between $78 billion to $80 billion versus $72.05 billion consensus analyst estimates. The Company observed 100% efficacy in Phase 2b trial of RSV adult vaccine candidate.
Conference Call Takeaways
Pfizer CEO Albert Bourla set the tone,
“Let me start with a commentary on some of our biggest growth drivers in the quarter. The Pfizer-BioNTech COVID-19 Vaccine contributed $7.8 billion in global revenues during the second quarter, and we continue to sign agreements with governments around the world. Just last week, we announced that the U.S. government has purchased an additional 200 million doses of the vaccine, bringing the total number of doses to be supplied to the U.S. government under its existing supply agreement to 500 million.
He continued:
"This is in addition to the 500 million doses that we agreed to provide to the U.S. government at a not-for-profit price, to be donated to the poorest countries in the world. We anticipate that a significant amount of the remaining 2021 vaccine manufacturing capacity will be delivered to middle and low-income countries, where we price in line with income levels or at a not-for-profit price.
"In fact, we are on track to deliver on our commitment to provide this year more than 1 billion doses, or approximately 40% of our total production to middle- and low-income countries and another 1 billion in 2022. Vyndaqel and Vyndamax revenues were up 77% operationally to $501 million globally.
"Our disease educational efforts in the U.S. continued to support increases in appropriate diagnosis, while the main driver of growth in Japan has been the successful establishment of several referral networks in select areas resulting in new patient starts. We anticipate these efforts will continue to support a strong trajectory for the franchise.
"Eliquis continued its strong performance, with revenues up 13% operationally to $1.5 billion. This was led by growth in the U.S. and emerging markets, driven primarily by the strength of the clinical profile, ease of use for both patients and clinicians, continued increased adoption in non-valvular atrial fibrillation, and overall oral anti-coagulant market share gains.
"Prevnar 13 in the U.S. was up 34% overall to $642 million. This growth was due primarily to higher levels of healthcare activity and wellness visits compared with the prior-year quarter, which was heavily impacted by COVID-19-related mobility restrictions and limitations.”
He concluded,
“Overall, I believe the second quarter was a clear and powerful demonstration of the capabilities of the new Pfizer. Looking forward, we intend to build upon these successes by continuing to follow the science, trust in our people, and remain focused on delivering breakthroughs for the patients we serve.
"As such, we continue to expect a revenue CAGR of at least 6%, on a risk-adjusted basis, through the end of 2025 and double-digit growth on the bottom line. I would note that these projections do not include any potential impact from our COVID-19 vaccine, recent or subsequent business development activities, or potential future mRNA programs. We remain very confident in our ability to achieve these growth rates because of the strength of both our current product portfolio and our R&D pipeline.”
COVID-19 Vaccine For Kids
On Oct. 22, 2021, Pfizer highlighted its Phase 2/3 study indicates a 90.7% efficacy rate for kids aged 5 to 12 years old. There were no cases of severe COVID-19 in 2,000 study participants after 2 months of receiving the second dose. The Company is currently awaiting a response on its request to the FDA to authorize a 10mg dosage for kids.
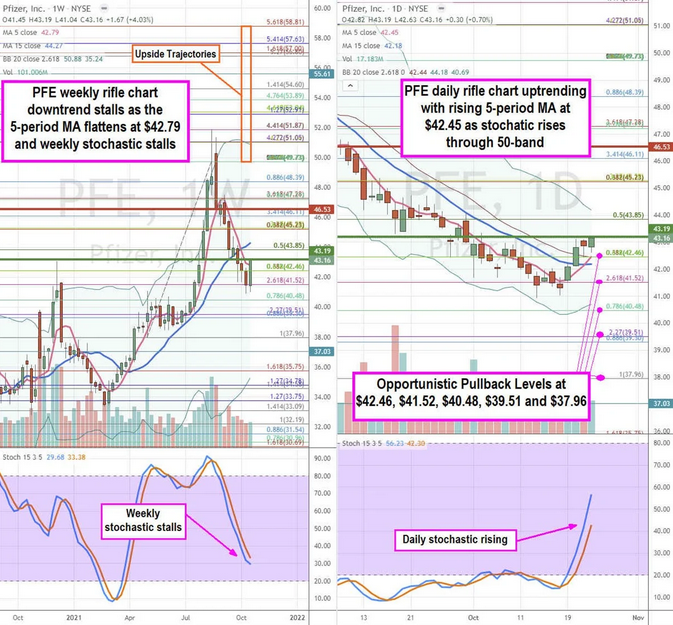
PFE Opportunistic Pullback Levels
Using the rifle charts on the weekly and daily time frames provides a precision view of the landscape for PFE stock. The weekly rifle chart peaked out near the $51.87 Fibonacci (fib) level. Shares sold off to a low of $40.94 before staging a rally. The weekly 5-period moving average (MA) resistance flattened at $42.79 followed by the 15-period MA at $44.27. It also formed a weekly market structure low (MSL) buy trigger on a breakout through $43.19. This caused the weekly stochastic to stall at the 30-band.
The daily rifle chart has since formed a breakout with a rising 5-period MA support at the $42.46 fib followed by the 15-period MA at $42.18. The daily stochastic oscillation is rising towards the 60-band.
Prudent investors can look for opportunistic pullback levels at the $42.46 fib, $41.52 fib, $40.48 fib, $39.51 fib, and the $37.96 fib. Upside trajectories range from the $49.73 fib upwards towards the $58.81 fib level.
Original Post
Trading in financial instruments and/or cryptocurrencies involves high risks including the risk of losing some, or all, of your investment amount, and may not be suitable for all investors. Prices of cryptocurrencies are extremely volatile and may be affected by external factors such as financial, regulatory or political events. Trading on margin increases the financial risks.
Before deciding to trade in financial instrument or cryptocurrencies you should be fully informed of the risks and costs associated with trading the financial markets, carefully consider your investment objectives, level of experience, and risk appetite, and seek professional advice where needed.
Fusion Media would like to remind you that the data contained in this website is not necessarily real-time nor accurate. The data and prices on the website are not necessarily provided by any market or exchange, but may be provided by market makers, and so prices may not be accurate and may differ from the actual price at any given market, meaning prices are indicative and not appropriate for trading purposes. Fusion Media and any provider of the data contained in this website will not accept liability for any loss or damage as a result of your trading, or your reliance on the information contained within this website.
It is prohibited to use, store, reproduce, display, modify, transmit or distribute the data contained in this website without the explicit prior written permission of Fusion Media and/or the data provider. All intellectual property rights are reserved by the providers and/or the exchange providing the data contained in this website.
Fusion Media may be compensated by the advertisers that appear on the website, based on your interaction with the advertisements or advertisers.