Jazz's (JAZZ) New Sleep Drug Sunosi Gets Approval In Europe
Zacks Investment Research | Jan 20, 2020 08:41PM ET
Jazz Pharmaceuticals plc (NASDAQ:JAZZ) announced that the European Commission approved its new sleep drug, Sunosi (solriamfetol). The drug will be available in Europe as a treatment to improve wakefulness and reduce excessive daytime sleepiness (“EDS”) in adults with narcolepsy (with or without cataplexy) or obstructive sleep apnea (“OSA”), whose EDS has not been satisfactorily treated by primary OSA therapy.
The approval was expected as the Committee for Medicinal Products for Human Use of the European Medicines Agency had recommended the drug’s approval in November 2019. Notably, Sunosi was approved by the FDA in March 2019 to improve wakefulness and reduce EDS in adult patients with narcolepsy or OSA.
With this approval, Sunosi becomes the first drug in Europe to treat EDS in adults living with OSA. Once daily two doses of Sunosi — 75 mg and 150 mg — is approved for narcolepsy patients while it will be available in three once daily doses — 37.5 mg, 75 mg and 150 mg — for OSA patients.
The marketing authorization for Sunosi in Europe was based on positive data from four clinical studies, including the TONES study, which demonstrated the superiority of Sunosi over placebo.
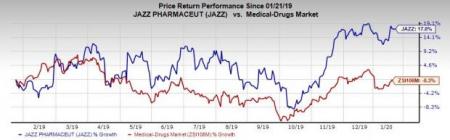
Jazz’s shares have gained 17% in the past year against the industry ’s decrease of 0.3%.
We note that the company has developed Sunosi in an indication similar to its key marketed drug, Xyrem. Jazz is also developing another candidate, JZP-258, a low sodium formulation and a Xyrem follow-on product, in late-stage studies for EDS and cataplexy in narcolepsy patients.
Xyrem sales grew significantly in 2018 and totaled $1.4 billion. In 2018, the drug generated almost 75% of Jazz’s total revenues. The company expects Xyrem’s sales to be in the range of $1.6-$1.64 billion in 2019. It is expected to report its fourth quarter earnings result next month. However, several patents protecting the drug are nearing expiration, starting December 2019. Moreover, an authorized generic version of the drug is likely to hit the markets as early as 2023.
The approval for Sunosi strengthened Jazz’s sleep disorder portfolio and is expected to help the company to offset a potential decline in Xyrem sales following a generic launch. Successful development of JZP-258 and its potential approval will be an additional boost for the company.
Approved drugs for narcolepsy include Teva Pharma’s (NYSE:TEVA) Provigil and Novartis’ (NYSE:NVS) Ritalin-SR. Some other pharma/biotech companies are developing treatments for narcolepsy. Avadel Pharmaceuticals plc (NASDAQ:AVDL) is one of them.

Trading in financial instruments and/or cryptocurrencies involves high risks including the risk of losing some, or all, of your investment amount, and may not be suitable for all investors. Prices of cryptocurrencies are extremely volatile and may be affected by external factors such as financial, regulatory or political events. Trading on margin increases the financial risks.
Before deciding to trade in financial instrument or cryptocurrencies you should be fully informed of the risks and costs associated with trading the financial markets, carefully consider your investment objectives, level of experience, and risk appetite, and seek professional advice where needed.
Fusion Media would like to remind you that the data contained in this website is not necessarily real-time nor accurate. The data and prices on the website are not necessarily provided by any market or exchange, but may be provided by market makers, and so prices may not be accurate and may differ from the actual price at any given market, meaning prices are indicative and not appropriate for trading purposes. Fusion Media and any provider of the data contained in this website will not accept liability for any loss or damage as a result of your trading, or your reliance on the information contained within this website.
It is prohibited to use, store, reproduce, display, modify, transmit or distribute the data contained in this website without the explicit prior written permission of Fusion Media and/or the data provider. All intellectual property rights are reserved by the providers and/or the exchange providing the data contained in this website.
Fusion Media may be compensated by the advertisers that appear on the website, based on your interaction with the advertisements or advertisers.