INSYS Gets Negative FDA Recommendation For Pain Candidate
Zacks Investment Research | May 22, 2018 10:22PM ET
INSYS Therapeutics, Inc. (NASDAQ:INSY) announced that an FDA advisory committee voted against the approval of its lead pipeline candidate, buprenorphine sublingual spray, for the treatment of moderate-to-severe acute pain.
The company had filed a new drug application ("NDA") in September last year based on positive data from a pivotal study on the candidate. The FDA accepted the NDA for review in December with a PDUFA date of Jul 28, 2018.
However, the unfavorable vote by the committee is expected to delay the approval of the drug as the FDA may ask for additional data for further evaluation.
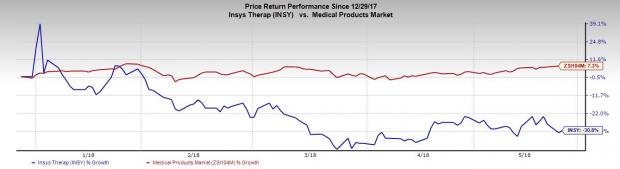
INSYS’ shares have declined 30.8% so far this year, underperforming the industry ’s decrease of 7.3%. We expect further decline in shares due to this unfavorable development for its lead pipeline candidate.
The NDA included data from a pivotal phase III study, which evaluated three dosing regimens (0.5mg, 0.25mg and 0.125 mg thrice per day) of buprenorphine sublingual spray versus a placebo in patients who were suffering from moderate to severe postoperative pain after bunionectomy. Data from the study showed that all the three doses achieved statistically significant improvement in pain from baseline after 48-weeks compared to placebo.
Apart from buprenorphine sublingual spray, INSYS has several other candidates in its pipeline including cannabidiol oral solution, naloxone nasal spray and buprenorphine/naloxone sublingual spray. The company is developing cannabidiol oral solution in phase III study for the treatment of infantile spasms and two phase II studies in Prader Willi syndrome and childhood absence epilepsy. The other two candidates are in early stages of development.

Trading in financial instruments and/or cryptocurrencies involves high risks including the risk of losing some, or all, of your investment amount, and may not be suitable for all investors. Prices of cryptocurrencies are extremely volatile and may be affected by external factors such as financial, regulatory or political events. Trading on margin increases the financial risks.
Before deciding to trade in financial instrument or cryptocurrencies you should be fully informed of the risks and costs associated with trading the financial markets, carefully consider your investment objectives, level of experience, and risk appetite, and seek professional advice where needed.
Fusion Media would like to remind you that the data contained in this website is not necessarily real-time nor accurate. The data and prices on the website are not necessarily provided by any market or exchange, but may be provided by market makers, and so prices may not be accurate and may differ from the actual price at any given market, meaning prices are indicative and not appropriate for trading purposes. Fusion Media and any provider of the data contained in this website will not accept liability for any loss or damage as a result of your trading, or your reliance on the information contained within this website.
It is prohibited to use, store, reproduce, display, modify, transmit or distribute the data contained in this website without the explicit prior written permission of Fusion Media and/or the data provider. All intellectual property rights are reserved by the providers and/or the exchange providing the data contained in this website.
Fusion Media may be compensated by the advertisers that appear on the website, based on your interaction with the advertisements or advertisers.