Akari's Nomacopan Gets Orphan Drug Tag For Rare Skin Disease
Zacks Investment Research | Sep 15, 2019 10:29PM ET
Akari Therapeutics, Plc (NASDAQ:AKTX) announced that the FDA has granted an orphan drug designation to its lead pipeline candidate nomacopan for the treatment of bullous pemphigoid (“BP (LON:BP)”). Currently, there is no approved therapy for this rare disease, which causes severe blistering of the skin. The status makes nomacopan eligible for seven years of marketing exclusivity in the United States.
Notably, the orphan drug designation is granted to drugs capable of treating rare diseases that affect less than 200,000 people in the United States. This designation also makes the company entitled to certain other benefits including tax credits related to clinical study expenses and an exemption from certain administrative fees.
The company is currently evaluating the candidate in a phase II study for treating patients with BP. In April, it had reported positive initial data from this study.
Please note that nomacopan was granted orphan drug designation for the treatment of hematopoietic stem cell transplantation-associated thrombotic microangiopathy (HSCT-TMA) by the FDA last month.
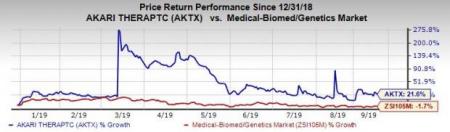
Shares of Akari have increased 21.6% so far this year against the industry ’s decline of 1.7%.
Nomacopan is a C5 complement inhibitor that specifically inhibits leukotriene B4 (LTB4) activity. Complement component C5, the target of nomacopan, is mostly found in the blood. Pre-clinical studies on nomacopan demonstrated that it is significantly more effective than LTB4 or C5 alone. Currently marketed LTB4 and C5 drugs include Zileuton and Alexion Pharmaceuticals’ (NASDAQ:ALXN) Soliris, respectively.
Apart from BP, the company is developing the candidate in three other rare diseases — HSCT-TMA, atopic keratoconjunctivitis ("AKC") and paroxysmal nocturnal hemoglobinuria ("PNH").
Nomacopan is in most advanced stage of development as a treatment for HSCT-TMA in pediatric patients. The company is planning to initiate a pivotal phase III study for this indication in the fourth quarter of 2019. Meanwhile, the company has successfully completed a phase II study, evaluating the candidate in PNH patients.
With no approved products, the company is dependent on successful development of nomacopan.

Trading in financial instruments and/or cryptocurrencies involves high risks including the risk of losing some, or all, of your investment amount, and may not be suitable for all investors. Prices of cryptocurrencies are extremely volatile and may be affected by external factors such as financial, regulatory or political events. Trading on margin increases the financial risks.
Before deciding to trade in financial instrument or cryptocurrencies you should be fully informed of the risks and costs associated with trading the financial markets, carefully consider your investment objectives, level of experience, and risk appetite, and seek professional advice where needed.
Fusion Media would like to remind you that the data contained in this website is not necessarily real-time nor accurate. The data and prices on the website are not necessarily provided by any market or exchange, but may be provided by market makers, and so prices may not be accurate and may differ from the actual price at any given market, meaning prices are indicative and not appropriate for trading purposes. Fusion Media and any provider of the data contained in this website will not accept liability for any loss or damage as a result of your trading, or your reliance on the information contained within this website.
It is prohibited to use, store, reproduce, display, modify, transmit or distribute the data contained in this website without the explicit prior written permission of Fusion Media and/or the data provider. All intellectual property rights are reserved by the providers and/or the exchange providing the data contained in this website.
Fusion Media may be compensated by the advertisers that appear on the website, based on your interaction with the advertisements or advertisers.