Dollar in demand, euro slumps after U.S.-EU trade agreement
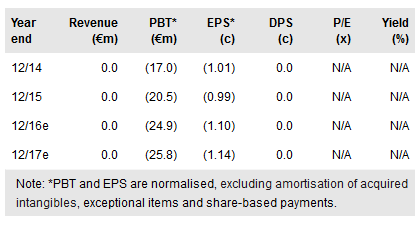
Mologen’s strategic review has focused its efforts on the development and commercialisation of late and early clinical assets, notably lead candidate lefitolimod (oncology and HIV) and EnanDIM, a next-generation TLR9 agonist. Partnering opportunities for lefitolimod are being actively sought; we anticipate Phase III data in 2018. Manufacturing efforts will be out-sourced to achieve suitable scale. MGN1601 (renal cancer vaccine) is on hold. Mologen’s move to a more clinical development-focused company means preclinical R&D assets (MIDGE vector system) will be spun off or divested. We value Mologen at €201m or €8.87/share.
Lefitolimod the near-term focus
Lefitolimod, a TLR9 agonist, is in four clinical trials, notably Phase III (IMPALA) and Phase II (IMPULSE) maintenance therapy trials for mCRC and SCLC respectively. IMPALA is expected to finish enrolment (c 540 patients) by year end (data expected in 2018), while the IMPULSE trial is expected to read out results in early/mid-2017. The expansion arm of the TEACH, Phase I trial for HIV and a separate Phase I combination trial with ipilimumab (Yervoy, BMS) recently enrolled first patients.
To read the entire report Please click on the pdf File Below