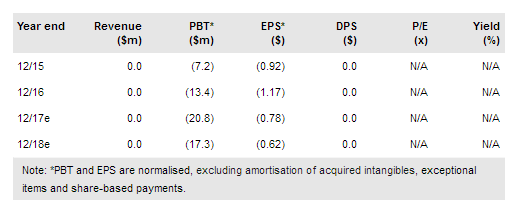
Intec Pharma Ltd (NASDAQ:NTEC) announced on 3 August 2017 that it had completed the Phase I clinical trial of AP-CBD/THC in 21 healthy volunteers. AP-CBD/THC is a formulation of cannabidiol and tetrahydrocannabinol using Intec’s proprietary accordion pill technology. The trial compared its safety, tolerability, and pharmacokinetics to the buccal cannabis extract, Sativex. AP-CBD/THC showed improved exposure, time at peak, and metabolism compared to Sativex without any safety or tolerability concerns.
Exposure and time at peak increased over Sativex
Intec announced that AP-CBD/THC improved the exposure of CBD by 290% to 330% and THC by 25% to 50% compared to Sativex. This demonstrates that the AP formulation of these molecules can deliver these molecules to patients at pharmacologically relevant concentrations. Additionally, the time at peak concentration was 2-3x longer, suggesting that the gastroretentive properties of the AP formulation are effective at prolonging the dissolution of the drug.
To read the entire report Please click on the pdf File Below:
Which stock should you buy in your very next trade?
AI computing powers are changing the stock market. Investing.com's ProPicks AI includes 6 winning stock portfolios chosen by our advanced AI. In 2024 alone, ProPicks AI identified 2 stocks that surged over 150%, 4 additional stocks that leaped over 30%, and 3 more that climbed over 25%. Which stock will be the next to soar?
Unlock ProPicks AI