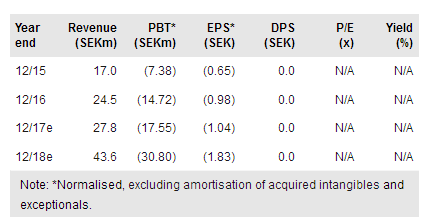
Immunovia publ AB (ST:IMMNOV) is on track to start commercialising its PanCan-d test for the early detection of pancreatic cancer in high-risk patients in 2018. To this end, it has established its US headquarters in Boston, which includes a laboratory regulated via the Clinical Laboratory Improvement Amendments (CLIA) programme. It has also identified a new important opportunity for the PanCan-d test in the early pancreatic cancer symptoms group, has announced further positive data on the differentiation of autoimmune diseases, and continues the preparations for a retrospective study and a biomarkers study in the diabetic population. Our valuation is SEK157/sh.
First PanCan-d sales on track for 2018
As previously guided, Immunovia plans to start out-of-pocket sales in 2018 in the US (as a CLIA-accredited laboratory developed test) and the EU (CE mark). It will seek reimbursement after the PANFAM-1 study, a three-year prospective clinical trial in 1,000 high-risk individuals with results expected in 2019. Immunovia aims to have PanCan-d included in surveillance programmes in familial pancreatic cancer and we estimate US and EU sales could represent a SEK2bn opportunity.
To read the entire report Please click on the pdf File Below:
Which stock should you buy in your very next trade?
With valuations skyrocketing in 2024, many investors are uneasy putting more money into stocks. Unsure where to invest next? Get access to our proven portfolios and discover high-potential opportunities.
In 2024 alone, ProPicks AI identified 2 stocks that surged over 150%, 4 additional stocks that leaped over 30%, and 3 more that climbed over 25%. That's an impressive track record.
With portfolios tailored for Dow stocks, S&P stocks, Tech stocks, and Mid Cap stocks, you can explore various wealth-building strategies.